From Technology
to Treatment
Advancing Precision Medicine
28 & 29 August 2025
ETH Zurich, Main Building







About the Event
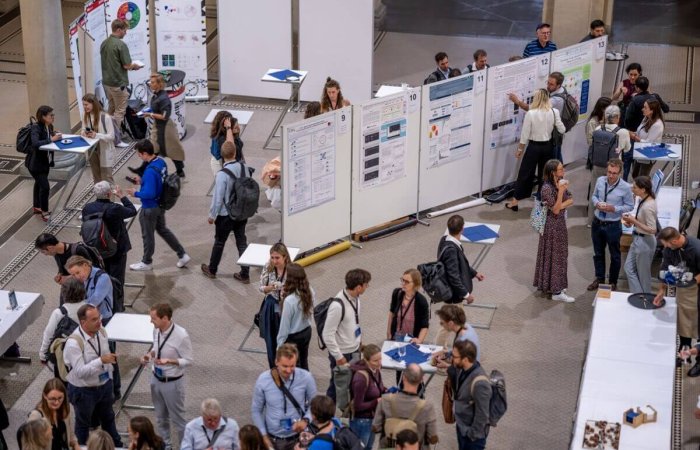
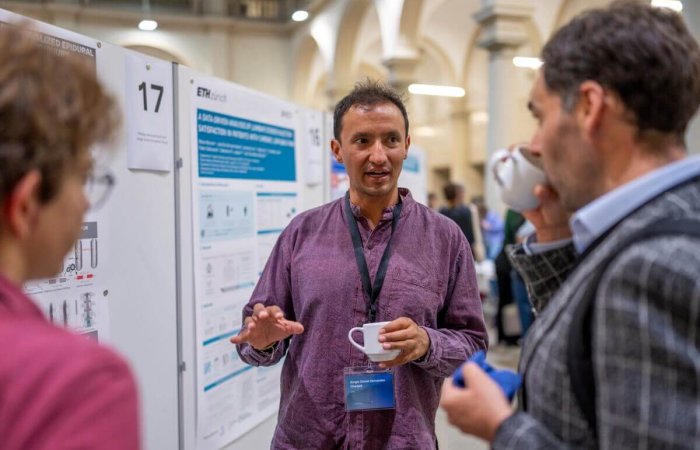
The From Technology to Treatment conference, co-organized by PHRT and SPHN, brought together over 300 participants from research, clinical practice, and innovation. With dynamic keynotes, insightful discussions, and engaging poster sessions, the event showcased the power of collaboration in advancing personalized health.
Explore the highlights, revisit key moments, and stay connected as we continue shaping the future of precision medicine—together.
Key Highlights:
- Keynote lectures on the latest advances in personalized medicine in Switzerland and abroad.
- A panel discussion with renowned leaders from academia, healthcare, patients and industry, addressing the current state and future opportunities of personalized medicine.
- Presentations of groundbreaking projects that are translating research into real-world clinical applications.
- Opportunities to explore national and international collaborations and synergies that will advance the field.

PHRT & SPHN
The PHRT – SPHN Event 2025 brought together leading researchers, clinicians, policymakers, and industry experts to showcase the advancements in Personalized Medicine and Health in Switzerland. This event served as a platform to highlight the impact of PHRT – Personalized Health & Related Technologies and Swiss Personalized Health Network (SPHN), fostering collaboration and innovation in the field.
#FromTechtoTreatment
Presentations
-
Precision Medicine Transforming Healthcare
- Mark Caulfield -
Reflecting on 8 Years of Progress An Overview of the PHRT and SPHN Initiatives
- Matthias Baumgartner & Bernd Wollscheid -
Advancing Precision Medicine with integrated Multi-Omics Approaches
- Sandra Goetze -
Charting Cellular Plasticity through Multi-Omics Profiling and Data Integration
- Marija Buljan -
SPO-NDS Fueling Precision Oncology in Switzerland
- Olivier Michielin -
From Data to Decisions Leveraging the National IICU Data Stream
- Catherine Jutzeler -
DigiSante Transforming Swiss Healthcare Through Digitalization
- Katrin Crameri -
The Rapid01 Trial Personalized Therapy for Acute Myeloid Leukemia Patients
- Alexandre Theocharides -
The Rapid01 Trial Personalized Therapy for Acute Myeloid Leukemia Patients
- Berend Snijder
-
Imaging in Population Science and Digital Twinning for Precision Cardiology
- Adam Lewandowski -
Imaging in Population Science and Digital Twinning in Precision Cardiology
- Sebastian Kozerke -
Stop Data Sharing
- Barend Mons -
Predicting Clinical Outcomes from Patient Care Pathways Represended with Temporal Knowledge Graphs
- Adrien Coulet -
Exploring the Next Generation of Radiotheragnostics for Metastatic Prostate Cancer
- Roger Schibli -
Exploring the Next Generation of Radiotheragnostics for Metastatic Prostate Cancer
- Damian Wild -
Federated Learning from FAIR data
- Andre Dekker
Our Speakers
Prof. Sir Mark Caulfield
Vice Principal for Health, Director of the NIHR Barts Biomedical Research Centre, Former Chief Scientist at Genomics England
Queen Mary University of London

Abstract:
Precision Medicine – Transforming Healthcare
The UK 100,000 Genomes Project focussed on transforming genomic medicine in the National Health Service using whole genome sequencing in rare disease, cancer and infection. Whole genome sequencing is reading as much of entire genetic code that we can today. Genomics England partnering with the NHS to establish Genomic Medicine Centres, an NHS whole genome sequencing centre and the Genomics England Clinical Interpretation Partnership (3500 researchers from 33 countries). We sequenced the 100,000th genome on the 5th December 2018 and have returned results to the NHS. Alongside these genomes we have assembled a longitudinal life course dataset for research and diagnosis including > 4 billion clinical data points for researchers to work on to drive up the value of the genomes for direct healthcare. In parallel we have partnered the NHS to establish one of the world’s most advanced Genomic Medicine Services where we re-evaluated 300,000 genomic tests and upgraded 25% of tests to newer technologies with an annual review and 500,000 whole genomes are available for NHS care over the next 5 years.
Prof. Dr. Dean Ho
Provost’s Chair Professor, Director of the N.1 Institute for Health. Director of the Institute for Digital Medicine (WisDM), Head of the Department of Biomedical Engineering
National University of Singapore

Abstract:
Precision Begins With One
Prof. Dean Ho and his team pioneered the development of CURATE.AI, an AI-enabled platform that dynamically optimises human drug dosing for indications ranging from hematologic and solid cancers to cardiovascular medicine and digital therapy. CURATE.AI is currently being studied across multiple first-in-kind clinical trials which have led to promising clinical outcomes. Lessons learned from AI-driven, N-of-1 healthcare has also led to new studies pertaining to Digital Longevity Medicine to address the need to extend human healthspan.
In October of 2024, Prof. Dean Ho and team launched DELTA, a first-in-kind human trial – with Prof. Dean Ho as the test subject. This N-of-1 protocol harnesses a combination of AI, digital medicine, fasting, fitness, and food to optimize metabolic health, monitored using an array of digital health platforms. Built from an unprecedented dataset, this study will culminate in a digital twin of Dean to hyper-personalise his cardiometabolic health protocol. Outcomes from this trial will create data collection frameworks to power population-scale healthspan optimisation and design regimens that do not require sustained digital monitoring to impact even larger communities.
Prof. Dr. Adam Lewandowski
Deputy Chief Scientist for UK Biobank and Associate Professor at University of Oxford
UK Biobank

Abstract:
Imaging in population science and digital twinning for precision cardiology
The talk will be held jointly with Prof. Dr. Sebastian Kozerke
Population-scale imaging studies, utilising non-invasive modalities such as MRI, provide critical insights into cardiovascular structure, function, and subclinical disease phenotypes across diverse demographics. These large datasets enable researchers to uncover novel biomarkers, model disease trajectories, and assess genetic and environmental risk factors with unprecedented granularity. By linking imaging data to clinical records, genomics, and lifestyle information, population science lays the foundation for predictive and preventive strategies in cardiovascular care as highlighted by the numerous landmark studies of the UK Biobank.
Based on the successes of the UK biobank, PHRT SwissHeart is leveraging population-scale imaging to derive functional and personalized digital twin hearts, thereby boosting the capacity for disease detection and prediction based on mechanistic and statistical/ML modelling. These digital twins are tailored not only to current health status and data, but will also permit improved projections of pathways of disease progression as well as restoration of health in the individual subject. It is the dynamic integration and augmentation of patient’s data that constitutes a key element of precision medicine as it will be highlighted in the talk.
Prof. Dr. Sebastian Kozerke
Professor at the Department of Information Technology and Electrical Engineering
ETHZ

Abstract:
Imaging in population science and digital twinning for precision cardiology
The talk will be held jointly with Prof. Dr. Adam Lewandowski
Population-scale imaging studies, utilising non-invasive modalities such as MRI, provide critical insights into cardiovascular structure, function, and subclinical disease phenotypes across diverse demographics. These large datasets enable researchers to uncover novel biomarkers, model disease trajectories, and assess genetic and environmental risk factors with unprecedented granularity. By linking imaging data to clinical records, genomics, and lifestyle information, population science lays the foundation for predictive and preventive strategies in cardiovascular care as highlighted by the numerous landmark studies of the UK Imaging Biobank.
Based on the successes of the UK imaging biobank, PHRT SwissHeart is leveraging population-scale imaging to derive functional and personalized digital twin hearts, thereby boosting the capacity for disease detection and prediction based on mechanistic and statistical/ML modelling. These digital twins are tailored not only to current health status and data, but will also permit improved projections of pathways of disease progression as well as restoration of health in the individual subject. It is the dynamic integration and augmentation of patient’s data that constitutes a key element of precision medicine as it will be highlighted in the talk.
Prof. Dr. Barend Mons
Founding director of the Leiden Institute for FAIR and Equitable Science (LIFES)
Leiden University, the Netherlands

Abstract:
Stop data sharing
The rapid developments in the field of machine learning have also brought along some existential challenges, which are in essence all related to the broad concept of ‘trust’. Aspects of this broad concept include trust in the output of any ML process (and the prevention of black boxes, hallucinations and so forth). The very trust in science is at stake, especially now that paper mills come up that also aggravate the perverse reward systems in current research environments, which are stuck in 20th (in fact 17th) century scholarly communication. The other side of the same coin is that ML, if not properly controlled, will also break through security and privacy barriers and violate GDPR and other Ethical, Legal and Societal barriers, including equitability. In addition, the ‘existence’ of data somewhere by no means implies its actual Reusability. This includes the by now well established four elements of the FAIR principles: Much data is not even Findable, if found, not Accessible under well defined conditions, and if accessed not Interoperable (understandable by third parties and machines) and this results in the vast majority of data and information not being Reusable without violation of copyrights, privacy regulations or the basic conceptual models that implicitly or explicitly underpin the query or the deep learning algorithm. This keynote will address how ‘data visiting’ as opposed to classical ‘data sharing’, which carries the connotation of data downloads, transport and losing control, mitigates most, if not all, the unwanted side effects of classical ‘data sharing’. For federated data visiting, the data should be FAIR in an additional sense or perspective, they should be ‘Federated, AI-Ready’, so that visiting algorithms can answer questions related to Access Control, Consent, Format, and can read rich (FAIR) metadata about the data itself to determine whether they are ‘fit for purpose’ and machine actionable (i.e. FAIR digital Objects, or Machine Actionable Units). The ‘fitness for purpose’ concept goes way beyond (but includes) information about methods, quality, error bars etc. The ‘immutable logging’ of all operation of visiting algorithms is crucial, especially when self learning algorithms in ‘swarm learning’ are being used. Enough to keep us busy for a while.
Prof. Dr. Antoine Geissbühler
Dean of the faculty of medicine, University of Geneva; Director, teaching and research.
Geneva University Hospitals
Prof. Dr. Andre Dekker
Professor of Clinical Data Science and board certified Medical Physicist
Maastricht University, the Netherlands

Abstract:
Federated Learning from FAIR data
Artificial intelligence, big data, machine learning and data science are expected to have a major impact on day-to-day health care with the first AI products already available to the medical community. But to be successful, these innovations need lots of data to be developed and validated and getting access to sufficient data is hampered by administrative, political, ethical and technical barriers. Since 2007, Maastricht University has embarked on an R&D program to build a global federated, privacy-preserving, FAIR (Knowledge Graph / RDF-based) data infrastructure called the Personal Health Train. The rationale, challenges and results of this infrastructure will be discussed.
Dr. Marija Buljan
Research Group Lead
Empa, Group Multi-omics for healthcare materials, SIB
Abstract:
Charting cellular plasticity through multi-omics profiling and data integration
Depending on the context and stimuli they receive, immune cells can adopt a plethora of different functional states and have a decisive role in keeping healthy homeostasis or promoting disease development. In our work, we expose primary human macrophages and other immune cells to physiologically relevant stimuli and perform in-depth characterization of different cellular states using transcriptomic, mass spectrometry-based proteomic and phosphoproteomic profiling. We leverage the generated phosphoproteome profiles to infer central regulatory signalling events and highlight individual kinases based on the direct and indirect footprints of their activities. Furthermore, by studying cellular transcriptome profiles, we find instances where the generated in vitro cellular states correspond to cell populations present in the tumours of cancer patients characterized with single cell transcriptomics. Interestingly, differentially regulated genes and proteins in macrophages exposed either to signalling mediators that are abundant in tumour microenvironment or directly to media collected from tumour resections cultured in vitro frequently point to the combined presence of inflammatory and immunosuppressive molecular signatures in the same cell states. In order to integrate the generated multi-omics datasets, we construct cellular networks by mapping representative entities for each omics layer onto knowledge-based protein interactions. We use these maps to identify and describe central regulatory elements, which connect multiple entities altered in the studied phenotypes and we leverage MONET-based network decomposition to highlight functionally connected network modules. The latter approach enables identification of cell state-specific and disease-relevant modules across the plastic phenotypic landscapes. Apart from the here described application to charting regulatory routes in cellular models, we demonstrate that this approach is also of a high value for the analysis of clinical multi-omics datasets.
Dr. Adrien Coulet
Researcher and co-leader of the interdisciplinary HeKA team
L’institut national de recherche en sciences et technologies du numérique (Inria), France

Abstract:
Predicting clinical outcomes from patient care pathways represented with temporal knowledge graphs
Background: With the increasing availability of healthcare data, predictive modeling finds many applications in the biomedical domain, such as the evaluation of the level of risk for various conditions, which in turn can guide clinical decision making. However, it is unclear how knowledge graph data representations and their embedding, which are competitive in some settings, could be of interest in biomedical predictive modeling.
Method: We simulated synthetic but realistic data of patients with intracranial aneurysm and experimented on the task of predicting their clinical outcome. We reduced this task to the classification of a subtype of nodes, and as a baseline, we evaluated its performance on tabular data. Next, we generated various graph-based representations of the same dataset, including a representation following the schema proposed by the SPHN (Swiss Personalized Healthcare Network), and investigated how the adopted schema for representing first individual data and second temporal data impacts predictive performances.
Results: Our study illustrates that in our case, the SPHN graph representation, along with Graph Convolutional Network (GCN) embeddings reach the best performance for a predictive task from observational data. We emphasize the importance of the adopted schema and of the consideration of literal values in the representation of individual data. Our study also moderates the relative impact of various time encoding on GCN performance.
Availability: This work has been accepted for publication and presentation to the Research track of the Extended Semantic Web Conference 2025. The preprint of the article is available at https://arxiv.org/abs/2502.21138.
Prof. Dr. Catherine Jutzeler
Assistant Professor at the Department of Health Sciences and Technology
ETHZ, SIB

Abstract:
From Data to Decisions: Leveraging the National IICU Data Stream
Infection-related outcomes in the ICU are influenced by complex interactions between host factors, pathogens, and clinical interventions. To address this heterogeneity, the National IICU (Infection-Related Outcomes in Swiss ICUs) Data Stream has been developed, building upon the foundation of the Personalized Swiss Sepsis Study (PSSS). The scope has been extended beyond sepsis to support personalized, data-driven care for a wider range of infectious conditions. This initiative integrates high-resolution, multi-source clinical data with standardized annotation of infection phenotypes, interventions, and outcomes. It emphasizes contextual understanding—capturing not just what was done, but why—by combining expert clinical input with structured ontologies. The platform follows FAIR principles to ensure long-term usability and interoperability across institutions. In this presentation, I will introduce the structure and aims of the IICU Data Stream, report on key lessons learned, including challenges in data harmonization, clinical context capture, and balancing algorithmic and expert-based phenotype interpretation. These insights have directly informed the design of the current infrastructure. Ultimately, the IICU Data Stream seeks to enable real-time, individualized decision support, support infection-related research, and improve outcomes in critical care. It serves as a model for integrating clinical, microbiological, and data science expertise into a unified national resource.
Dr. Katrin Crameri, MPH
Co-Lead DigiSanté, Federal Department of Home Affairs
Digital Transformation Division, Swiss Federal Office of Public Health (FOPH)

Abstract:
DigiSanté – Transforming Swiss Healthcare Through Digitalization
The Swiss healthcare system has long been hindered by fragmented data solutions, proprietary systems, and a lack of fundamental digital transformation. DigiSanté, a federal initiative launching in 2025 with a ten-year mandate and a 392 million CHF budget, aims to address these challenges by establishing a standardized and interoperable digital infrastructure.
At the heart of DigiSanté is the Swiss Health Data Space (SwissHDS), designed to facilitate seamless, secure, and fully automated data exchange across healthcare providers, insurers, research institutions, and public health authorities. Through harmonized standards, specifications, and interfaces, SwissHDS will enable more efficient primary healthcare processes while ensuring responsible secondary use of health data for research, planning, and system management.
This presentation will explore the vision, key objectives, and strategic roadmap of DigiSanté. We will discuss how the program seeks to overcome the historical silos in Swiss healthcare, implement cross-cutting legislation for digital health infrastructure, and drive the adoption of a national strategy that balances innovation with governance.
By embracing change, fostering collaboration among stakeholders, and enforcing digital standards, DigiSanté has the potential to reshape Swiss healthcare for the future.
Abstract:
Exploring the next Generation of Radiotheragnostics for metastatic Prostate Cancer
The talk will be held jointly with Prof. Dr. Roger Schibli
Radioligand therapy (RLT) has emerged as an effective treatment for patients with progressive, prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC). Despite the success of RLT, approximately one-third of patients do not respond or experience early relapse. We hypothesize that suboptimal dose delivery to microscopic disease, including single and clustered circulating tumor cells (CTCs), may contribute to treatment resistance.
Our PROGNOSTICS Phase I study (NCT06343038) investigates the efficacy, safety, and dosimetry of [¹⁶¹Tb]Tb-SibuDAB—a novel PSMA-targeting radioligand developed at the Paul Scherrer Institute—in comparison with the current standard RLT. Designed to overcome the limitations of existing PSMA-targeted therapies, [¹⁶¹Tb]Tb-SibuDAB has a long blood-circulation and consequently a higher tumor uptake and delivers not only β¯-particles but also a high dose to microscopic disease through the co-emission of conversion and Auger electrons. In this ongoing Phase Ia/b trial, mCRPC patients receive test injections with [¹⁶¹Tb]Tb-SibuDAB and the current standard compound [¹⁷⁷Lu]Lu-PSMA-I&T in a randomized cross-over design to compare tumor and organ dosimetry. The results are supported by quantitative SPECT/CT imaging, liquid biopsy analyses of CTCs, and molecular marker profiling. Preliminary results from the Phase Ia study show that [¹⁶¹Tb]Tb-SibuDAB delivers more than twice the dose to tumors and exhibits a longer tumor half-life compared to [¹⁷⁷Lu]Lu-PSMA-I&T, with favorable tumor-to-organ dose ratios and no relevant adverse effects. Early Phase Ib findings from the first three-patient cohort receiving therapeutic doses (3 GBq × 4 cycles) indicate a good safety profile. The study continues to evaluate optimal dosing, efficacy markers, and personalized predictors of response, aiming to establish [¹⁶¹Tb]Tb-SibuDAB as a next-generation radioligand therapy for mCRPC. This project exemplifies how the combined expertise within the ETH Domain and its clinical partners can deepen our molecular understanding of disease and contribute to improved treatment outcomes for cancer patients in the future.
Abstract:
Exploring the next Generation of Radiotheragnostics for metastatic Prostate Cancer
The talk will be held jointly with Prof. Dr. Dr. Damian Wild
Radioligand therapy (RLT) has emerged as an effective treatment for patients with progressive, prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC). Despite the success of RLT, approximately one-third of patients do not respond or experience early relapse. We hypothesize that suboptimal dose delivery to microscopic disease, including single and clustered circulating tumor cells (CTCs), may contribute to treatment resistance.
Our PROGNOSTICS Phase I study (NCT06343038) investigates the efficacy, safety, and dosimetry of [¹⁶¹Tb]Tb-SibuDAB—a novel PSMA-targeting radioligand developed at the Paul Scherrer Institute—in comparison with the current standard RLT. Designed to overcome the limitations of existing PSMA-targeted therapies, [¹⁶¹Tb]Tb-SibuDAB has a long blood-circulation and consequently a higher tumor uptake and delivers not only β¯-particles but also a high dose to microscopic disease through the co-emission of conversion and Auger electrons. In this ongoing Phase Ia/b trial, mCRPC patients receive test injections with [¹⁶¹Tb]Tb-SibuDAB and the current standard compound [¹⁷⁷Lu]Lu-PSMA-I&T in a randomized cross-over design to compare tumor and organ dosimetry. The results are supported by quantitative SPECT/CT imaging, liquid biopsy analyses of CTCs, and molecular marker profiling. Preliminary results from the Phase Ia study show that [¹⁶¹Tb]Tb-SibuDAB delivers more than twice the dose to tumors and exhibits a longer tumor half-life compared to [¹⁷⁷Lu]Lu-PSMA-I&T, with favorable tumor-to-organ dose ratios and no relevant adverse effects. Early Phase Ib findings from the first three-patient cohort receiving therapeutic doses (3 GBq × 4 cycles) indicate a good safety profile. The study continues to evaluate optimal dosing, efficacy markers, and personalized predictors of response, aiming to establish [¹⁶¹Tb]Tb-SibuDAB as a next-generation radioligand therapy for mCRPC. This project exemplifies how the combined expertise within the ETH Domain and its clinical partners can deepen our molecular understanding of disease and contribute to improved treatment outcomes for cancer patients in the future.
A. Chirindel1, G.P. Nicolas1, F. Westerbergh1, D. Schmid2, N. Ahmadsei1, L. McDougall1, A. Bauman8, S. Geistlich2, A. Fokkema3, M. Saini3, N.P. van der Meulen2,5, C. Müller2,7, P. Bernhardt2,6, N. Aceto3, D. Wild1, R. Schibli2,7
1 Division of Nuclear Medicine, University Hospital Basel, Switzerland
2 Center for Radiopharmaceutical Sciences, Paul Scherrer Institute (PSI), Villigen-PSI, Switzerland
3 Department of Biology, Institute of Molecular Health Sciences, ETH Zurich, Zurich, Switzerland
4 Department of Medical Radiation Sciences, Institute of Clinical Sciences, Sahlgrenska Academy at University of Gothenburg, Gothenburg, Sweden
5 Laboratory of Radiochemistry, Paul Scherrer Institute (PSI), Villigen-PSI, Switzerland
6 Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden
7 Department of Chemistry and Applied Biosciences, ETH Zurich, Zurich, Switzerland
8 Division of Radiopharmaceutical Chemistry, University Hospital Basel, Basel, Switzerland
Prof. Dr. Olivier Michielin
Head of Oncology and the Precision Oncology Service
Geneva University Hospital, SIB

Abstract:
SPO-NDS: Fueling Precision Oncology in Switzerland
“Multidimensional datasets are playing an increasingly important role in guiding treatment decisions in precision oncology. The Swiss Personalised Oncology (SPO) initiative aims to integrate clinical, molecular, and imaging data to support decision-making within molecular tumor boards. To this end, a national infrastructure has been established to collect and harmonize interoperable data across all Swiss University Hospitals and selected non-University Centres. In parallel, standardized multi-omics workflows have been implemented across these institutions to support treatment recommendations at both local and national levels. Illustrative examples will be presented to demonstrate how the integration of clinical, imaging, and multi-omics data can inform therapeutic strategies and improve patient care.”
PD Dr. Sean Froese
Head of Research Division Metabolism
University Children's Hospital Zurich
Abstract:
Enhancing rare disease diagnosis through multi-omics
Rare diseases comprise over 7’000 disorders, affecting approximately 400 million people worldwide and 500’000 individuals in Switzerland. Due to their typically early and severe onset, rare diseases disproportionately affect children, often leading to premature death or lifelong disability. Current diagnostic procedures are slow, low-yield, and offer limited insight into disease pathogenesis—highlighting the urgent need for new approaches
In partnership with the PHRT and the Swiss Multi-Omics Center (SMOC), we are pioneering rare diagnosis using multi-omics. Through whole genome sequencing (genomics), RNA sequencing (transcriptomics), and proteotyping (proteomics) alongside phenotypic and biochemical profiling (phenomics), we found a molecular diagnosis for 177 out of 210 cases of the rare metabolic disorder methylmalonic aciduria, and in 101 out of 150 individuals with a suspected remethylation disorder. Further investigations revealed a correlation between disease severity and disturbed energy metabolism in methylmalonic aciduria, evidenced also in cellular and animal models of disease, and disturbed translation in remethylation disorders. This multi-omics approach is now being used to modernize and improve rare disease diagnostic assays in our hospital.
As part of the National Data Stream SwissPedHealth, we are further developing a multi-omic workflow to diagnose rare diseases in critically ill children. We have initiated a Swiss-wide, prospective trial, encompassing all five Pediatric University Hospitals, which has already recruited over 75 patients. Genomic, transcriptomic, proteomic and metabolomic investigations in 37 individuals—including 23 undiagnosed by standard-of-care—illustrate both the challenges and opportunities of such an approach.
Overall, we are helping demonstrate the power of multi-omic based approaches to transform rare disease diagnosis, by increasing diagnostic rates and facilitating disease discovery, with the ultimate goal of supporting timely, life-saving therapies.
Prof. Dr. Alexandre Theocharides
Head of Clinical Studies CCCZ, Clinic for Medical Oncology and Haematology
University Hospital Zurich

Abstract:
With an overall survival of below 12 months, the outlook for patients suffering from relapsed or refractory acute myeloid leukemia (RR-AML) is very poor, making it critical to identify effective therapies for each patient. Over the past decade, we have developed an image-based drug screening approach (pharmacoscopy; PCY), which measures drug sensitivities directly in fresh patient samples to help identify effective individualized therapies for cancer patients. Previously, in two non-randomized proof-of-concept clinical studies, PCY has been shown to help identify treatments that lead to improved clinical responses in patients suffering from blood cancers. In this presentation, we will discuss the RAPID-01 trial, a randomized and controlled interventional clinical trial in which PCY is used to guide treatment selection for patients with RR-AML. The RAPID-01 trial is a highly innovative randomized and controlled Phase-2 in vitro diagnostic performance study, aiming to provide precision care for RR-AML patients in need. The ETH Zurich is the sponsor of the trial, with the University Hospital Zurich (USZ) as the lead clinical partner, and the Inselspital Bern as the second site currently recruiting patients. The RAPID-01 is helping to pave the way towards precision oncology in randomized controlled trials in Switzerland and beyond.
Prof. Dr. Berend Snijder
Assistant Professor/ Faculty Professor
ETHZ/ Botnar Institute of Immune Engineering (BIIE), SIB

Abstract:
With an overall survival of below 12 months, the outlook for patients suffering from relapsed or refractory acute myeloid leukemia (RR-AML) is very poor, making it critical to identify effective therapies for each patient. Over the past decade, we have developed an image-based drug screening approach (pharmacoscopy; PCY), which measures drug sensitivities directly in fresh patient samples to help identify effective individualized therapies for cancer patients. Previously, in two non-randomized proof-of-concept clinical studies, PCY has been shown to help identify treatments that lead to improved clinical responses in patients suffering from blood cancers. In this presentation, we will discuss the RAPID-01 trial, a randomized and controlled interventional clinical trial in which PCY is used to guide treatment selection for patients with RR-AML. The RAPID-01 trial is a highly innovative randomized and controlled Phase-2 in vitro diagnostic performance study, aiming to provide precision care for RR-AML patients in need. The ETH Zurich is the sponsor of the trial, with the University Hospital Zurich (USZ) as the lead clinical partner, and the Inselspital Bern as the second site currently recruiting patients. The RAPID-01 is helping to pave the way towards precision oncology in randomized controlled trials in Switzerland and beyond.
Abstract:
Advancing Precision Medicine with Integrated Multi-Omics Approaches
The Swiss Multi-Omics Center (SMOC) is a platform of the ETH Personalized Health and Related Technologies (PHRT) initiative that pioneers precision medicine by offering integrated multi-omics analysis of clinical samples. SMOC acts as a central hub, providing researchers and clinicians access to cutting-edge technologies and expert data interpretation to decipher the complex molecular basis of health and disease.
SMOC’s mission is to empower clinical research by generating molecular data, encompassing multiple omics layers, to facilitate a deeper understanding of disease mechanisms, enable biomarker discovery, targeted therapy development, and improved treatment response prediction. Capabilities of SMOC span genomics, transcriptomics, proteomics, and metabolomics. Services include ISO 15189-accredited Clinical Grade Sequencing, comprehensive quantitative and targeted proteomics, as well as targeted and untargeted metabolomics and lipidomics. SMOC is also developing computational algorithms and pipelines to extract meaningful insights from these vast multi-omics data collections. The mission of SMOC is further supported by strategic partnerships with the Swiss Personalized Health Network (SPHN), which advances ethical data infrastructure, and the Swiss Data Science Center (SDSC), which enhances predictive analytics across multi-dimensional datasets.
SMOC is actively involved in key research initiatives, including RAINDROP (Inborn Errors of Metabolism diagnosis), RAPIDS (pediatric sepsis diagnosis), and the tumor profiler. It contributes to the SwissPedHealth network’s pediatric research data stream and the “Genome of Switzerland” project, creating a national genomic reference dataset.
By facilitating access to advanced technologies and fostering collaboration, SMOC helps bridge the gap between research and clinical practice. It aims to ensure that multi-omics insights are both useful and practical for healthcare professionals thereby supporting a paradigm shift that tailors medical decisions to an individual’s unique molecular profile.
Prof. Dr. Matthias Baumgartner
Chair of the SPHN National Steering Board
Director Teaching and Research, University Children's Hospital Zurich
Prof. Dr. Bernd Wollscheid
Chair of the PHRT Executive Committee, Head of Institute of Translational Medicine / Head of Proteomics Platform D-HEST
PHRT & ETH Zurich
Event Agenda
Day 1: August 28, 2025
07:30 – 08:30
Arrival, Coffee, Registration
08:30 – 08:45
Welcome Address
Prof. Dr. Annette Oxenius
Vice President for Research at ETH Zurich
Prof. Dr. Arnaud Perrier
President SAMS
08:45 – 09:30
Precision Medicine – Transforming Healthcare
Prof. Sir Mark Caulfield (abstract)
Vice Principal for Health, Director of the NIHR Barts Biomedical
Research Centre at Queen Mary University of London & Former
Chief Scientist at Genomics England
Reflecting on 8 Years of Progress: An Overview of the PHRT and SPHN Initiatives
Prof. Dr. Matthias Baumgartner
SPHN National Steering Board Chair
Prof. Dr. Bernd Wollscheid
PHRT Executive Committee Chair
30 min – Coffee Break / Poster Exhibition & Booths
Multi-Omics: Mapping the Complexities of Life
10:30 – 11:00
Enhancing rare disease diagnosis through multi-omics
PD Dr. Sean Froese (abstract)
Head of Research Division Metabolism at University Children’s Hospital Zurich
11:00 – 11:15
Advancing Precision Medicine with Integrated Multi-Omics Approaches
Dr. Sandra Goetze (abstract)
PHRT Swiss Multi-Omics Center Coordinator
11:15 – 11:30
Charting cellular plasticity through multi-omics profiling and data integration
Dr. Marija Buljan (abstract)
Research Group Lead, Group Multi-omics for healthcare materials at Empa
11:30 – 12:00
Poster Pitches
60 min – Lunch Break / Poster Exhibition & Booths
Innovating PM: Systematic Data Generation for Impact
13:00 – 13:20
SPO-NDS: Fueling Precision Oncology in Switzerland
Prof. Dr. Olivier Michielin (abstract)
Head of Oncology and the Precision Oncology
Service at Geneva University Hospital
13:20 – 13:40
From Data to Decisions: Leveraging the National IICU Data Stream
Prof. Dr. Catherine Jutzeler (abstract)
Assistant Professor at the Department of Health Sciences and Technology at ETH Zurich
13:40 – 14:00
DigiSanté – Transforming Swiss Healthcare Through Digitalization
Dr. Katrin Crameri (abstract)
Co-Lead DigiSanté at the Swiss Federal Office of Public Health,
FOPH
30 min – Coffee Break / Poster Exhibition & Booths
Next Generation Technology: Redefining Possibilities
14:30 – 15:30
The RAPID-01 Trial: Personalized therapy for acute myeloid leukemia patients
Prof. Dr. Berend Snijder (abstract)
Assistant Professor at ETH Zurich & Faculty Professor at Botnar Institute of Immune Engineering
Prof. Dr. Alexandre Theocharides (abstract)
Head of Clinical Studies CCCZ at the Clinic for Medical Oncology and Haematology, University Hospital Zurich
15:30 – 16:15
Imaging in population science and digital twinning for precision cardiology
Prof. Dr. Adam Lewandowski (abstract) Deputy Chief Scientist for the UK Biobank and Associate Professor at University of Oxford
Prof. Dr. Sebastian Kozerke (abstract) Professor at the Department of Information Technology and Electrical Engineering at ETH Zurich
16:15 – 18:00
Apéro, Exhibition & Networking
Day 2: August 29, 2025
08:00 – 08:45
Arrival, Coffee
08:45 – 09:00
Welcome Address
Dr. Davide Chiarugi
Director of the SIB’s Personalized Health Informatics group, Technical Director SPHN
Dr. Daniel Vonder Mühll
Executive Director PHRT
09:00 – 10:00
Panel: From Discovery to Application: Navigating the Future of Personalized Medicine
PD Dr. Jasmin Barman-Aksözen
Scientist and patient advocate for rare disease, University of Zurich
Dr. Katrin Crameri
Co-Lead DigiSanté at the Swiss Federal Office of Public Health, FOPH
Prof. Dr. Antoine Geissbühler
Dean of the faculty of medicine, University of Geneva; Director, teaching and research at Geneva University Hospitals
Dr. Monika Jänicke
CEO & Chairwoman of the Hospital Management Board at University Hospital Zurich
Dr. Michael Rebhan
Senior Investigator at the Novartis Institutes for BioMedical
Research (NIBR) in Basel
Prof. Dr. Christian Rüegg
Director of PSI, Member of the ETH Board
30 min – Coffee Break / Poster Exhibition & Booths
FAIR Principles: Overcoming Challenges in Data Accessibility and Integration
10:30 – 11:00
Stop data sharing
Prof. Dr. Barend Mons (abstract)
Founding director of the Leiden Institute for FAIR and Equitable Science (LIFES), Leiden University, the Netherlands
11:00 – 11:30
Predicting clinical outcomes from patient care pathways represented with temporal knowledge graphs
Dr. Adrien Coulet (abstract)
Researcher and co-leader of the interdisciplinary HeKA team at Inria, France
90 min – Lunch Break / Poster Exhibition & Booths
Radiotheragnostics: Transforming Precision Medicine with Targeted Therapies
13:00 – 14:00
Exploring the Next Generation of Radiotheragnostics for Metastatic Prostate Cancer
Prof. Dr. Roger Schibli (abstract)
Head of Center for Radiopharmaceutical Sciences at PSI
Prof. Dr. Dr. Damian Wild (abstract)
Head of Nuclear Medicine at University Hospital Basel
Rahman Bajrami
Patient
20 min – Coffee Break / Poster Exhibition & Booths
Shaping Tomorrow: The Power of AI and Federated Computing
14:20 – 14:30
Appreciation
14:30 – 15:15
Precision Begins With One
Dr. Dean Ho (abstract)
Head of the Department of Biomedical Engineering at the National University of Singapore
15:15 – 16:00
Federated Learning from FAIR data
Prof. Dr. Andre Dekker (abstract)
Professor of Clinical Data Science at Maastricht University, the Netherlands
16:00 – 16:15
Poster Award / Acknowledgements / Closing Remarks
Prof. Dr. Bernd Wollscheid
Executive Committee Chair PHRT
Dr. Thomas Geiger
Managing Director SPHN
Achievements of PHRT & SPHN
500+ Researchers
A growing community
700,000+ Patients
Gave general consent
250+ Projects
Funded to advance precision medicine.
40+ Hospitals
Collaborating on research
Ethical & Legal Support
For multicenter studies
BioMedIT Platform
Trusted research environment
Location
ETH Zürich, Main Building, Switzerland